(BBC) -The first Covid-19 vaccination in the United States has taken place, as the country gears up for its largest ever immunisation campaign.
An intensive care nurse in Long Island, New York, is believed to have been the first person to be given the jab.
Millions of vials of the Pfizer/BioNTech vaccine are being distributed, with 150 hospitals expected to receive doses on Monday.
The US vaccination programme aims to reach 100m people by April.
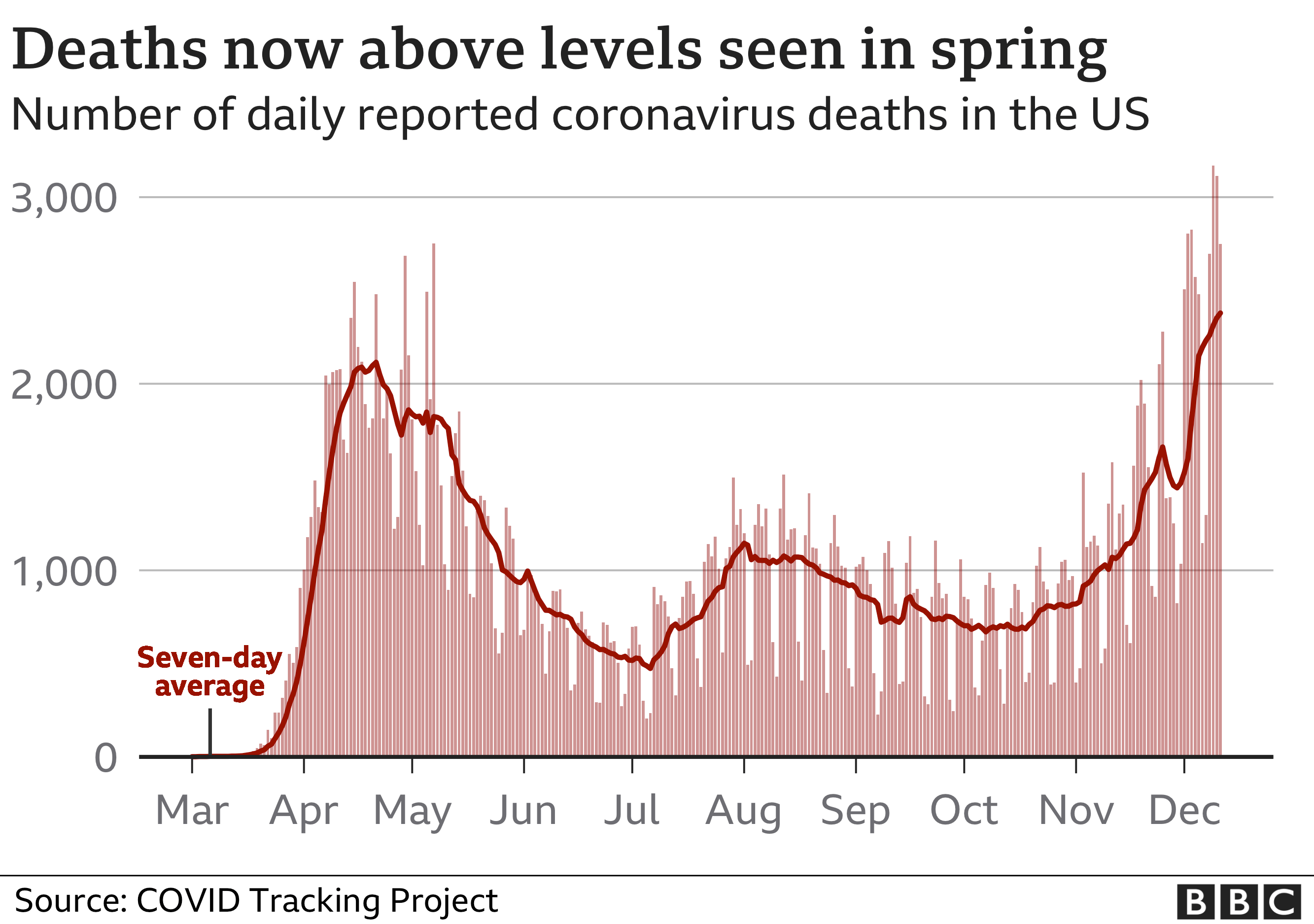
Covid-19 fatalities are nearing 300,000 in the US, which has by far the world’s highest death toll.
The Pfizer/BioNTech vaccine received emergency-use authorisation from the US Food and Drug Administration (FDA) on Friday.
“First Vaccine Administered. Congratulations USA! Congratulations WORLD!” President Donald Trump tweeted on Monday morning following the news from New York.
The roll-out of the vaccine comes as the epidemic continues to ravage the country. Deaths have been rising sharply since November and the number of people in hospital with the disease has also continued to grow steadily, with more than 109,000 people currently admitted, according to the Covid Tracking Project.
Sandra Lindsay, a nurse at Long Island Jewish Medical Center, received the vaccine live on-camera in a video that was streamed on the Twitter feed of New York Governor Andrew Cuomo, whose state was the epicentre of the country’s epidemic in the first wave earlier this year.
“It didn’t feel any different from taking any other vaccine,” Ms Lindsay said. “I hope this marks the beginning of the end of a very painful time in our history. I want to instil public confidence that the vaccine is safe. We’re in a pandemic and so we all need to do our part.”

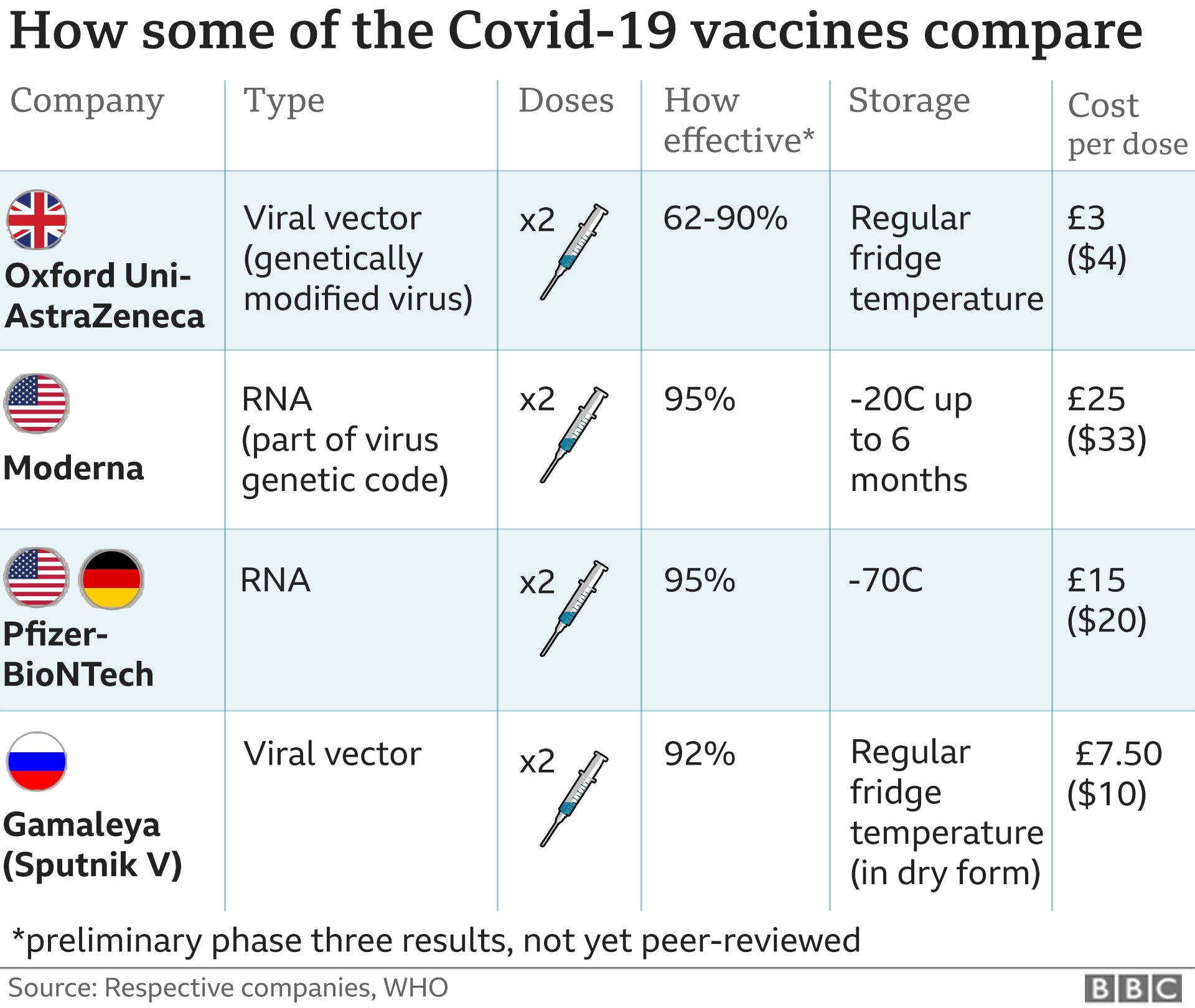
The Pfizer/BioNTech vaccine – a collaboration between a US pharmaceutical giant and a German biotechnology company – offers up to 95% protection and is the first Covid-19 vaccine to be approved by US regulators.
It is already being rolled out in the UK, while Canada is also beginning its inoculation programme on Monday, with an initial 30,000 doses going to 14 sites across the country.
The first three million doses in the US are being distributed to dozens of locations across all 50 states by cargo plane and truck.
Because the vaccine has to be kept at extremely low temperatures, the vials are stored in dry ice-cooled packages as they are whisked around the country. GPS-enabled thermal sensors are also being used to track the temperature of shipments as they are delivered.
Most Americans will not be able to receive the vaccine until well into 2021 but the roll-out beginning this week is seen as a key symbolic turning point in the nation’s battle against the pandemic, with hopes that take-up will be high.
“I think it’s been probably the darkest December on record here. As of this last week, Covid-19 is the leading cause of death in the US, even more than cancer and heart disease,” Dr Dora Mills of MaineHealth, a network of 12 hospitals in Portland, Maine, told the BBC
“It’s a very dark season for us, but it’s also extraordinary that we have a vaccine less than a year after this virus has emerged. If the efficacy and safety data hold up, this is likely [to be] the greatest public health and scientific achievement of our lifetime.”

The first doses are expected to be given to selected healthcare workers and elderly people living in residential care.
Senior members of the Trump administration had been due to be some of the first in line but President Trump now says he has reversed the plan.
He tweeted on Sunday that people working at the White House “should receive the vaccine somewhat later… unless specifically necessary”.
US President-elect Joe Biden, who will be inaugurated as president on 20 January, has set a goal of 100 million Covid vaccinations in his first 100 days in office.
That would represent roughly a third of the country’s total population.
Pfizer has agreed a deal to supply the US with 100 million doses of the vaccine by March.
An additional 200 million doses of a second vaccine, developed by Moderna and the National Institutes of Health, will be provided by June. The vaccine is yet to be approved by regulators but will be reviewed by an expert panel later this week.

